Engineered Co-polyesters
G. Gill (PSc 804)
15 July 2002
It may be useful to elucidate the structure and the names commonly used in describing these polymers. The polymer is made by condensing monomers of a hydroxyacid branched at C3.
The naming scheme is IUPAC starting with Polyhydroxypropylate (R=H). Abbreviated P(3HP), butyrate (R=CH3), valerate (R= C2H5), hexanoate (R=C3H7) , follow as the R group becomes larger (up to 13 Carbons), abbreviated as P(3HB), P(3HV), and P(3HHx). The first poly hydroxy alkanoid (PHA) to be discovered was P(3HB) by Lemoigne in 1926 and is therefore the widely studied.
Polyhydroxypropylate (R=H). Abbreviated P(3HP), butyrate (R=CH3), valerate (R= C2H5), hexanoate (R=C3H7) , follow as the R group becomes larger (up to 13 Carbons), abbreviated as P(3HB), P(3HV), and P(3HHx). The first poly hydroxy alkanoid (PHA) to be discovered was P(3HB) by Lemoigne in 1926 and is therefore the widely studied.
Interest in the production of bio-polyesters was initiated by W. R. Grace in the 1960s and later developed by Imperial Chemical Industries, Ltd., in the United Kingdom in the 1970s and 1980s, when there use as degradable surgical sutures was foreseen. Since the early 1990s, Metabolix Inc. and Monsanto have been manufacturing PHA polymers in the United States17,8
The main concern for degradability came with the environmental awareness prevalent in the 60s and 70s. However with biodegradability  the demand for polymers increases many fold with applications in medicine ranging from surgical sutures to implants and drug delivery packaging, the list grows as more and more variety of properties may be integrated through co-polymerization.
In fact the range of products as well as substrates grows exponentially as scientists research the metabolic pathways and the variety of combinations of transgenic strains and modifications to nutrient media. For example, Song and Yoon15 report production of a co polyester of (72-85%) 3-hydroxy 5-phenoxyvalerate and (15-28%) 3-hydroxy 7-phenoxyheptanoate, by P. putida grown on 11-phenoxyundecanoic acid. Our discussion will be limited to the production of P(3HB) and its co polymers with 3 valerate or 3 hexanoate.
the demand for polymers increases many fold with applications in medicine ranging from surgical sutures to implants and drug delivery packaging, the list grows as more and more variety of properties may be integrated through co-polymerization.
In fact the range of products as well as substrates grows exponentially as scientists research the metabolic pathways and the variety of combinations of transgenic strains and modifications to nutrient media. For example, Song and Yoon15 report production of a co polyester of (72-85%) 3-hydroxy 5-phenoxyvalerate and (15-28%) 3-hydroxy 7-phenoxyheptanoate, by P. putida grown on 11-phenoxyundecanoic acid. Our discussion will be limited to the production of P(3HB) and its co polymers with 3 valerate or 3 hexanoate.
Degradation of PHBV
The various ways in which a polymer may degrade are described above these conditions apply typically to PHBVs. Inside the cell the degradation is essentially the reverse of the synthesis, yielding acetyl acetate which gets incorporated back into the citric acid cycle.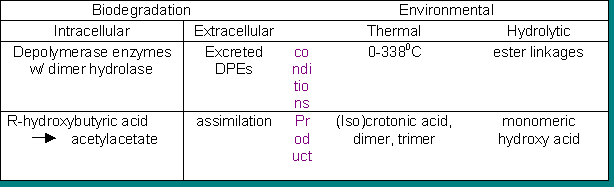 Depolymerase enzymes may be excreted by an organism so that the resulting monomers or dimers may be assimilated into the cell as a nutrient. Thermal degradation results mainly in the dimer ( 20 -38%), some trimer and small amounts of crotonic and iso-crotonic acids. A hydrolytic attack of the ester linkages results in the monomeric hydroxy-acid and is fairly rapid degrading, for example, a bottle is fully degraded within a few months. There are various alternatives to extending the time frame of degradation for example PHBV blend with 75% Cellulose, acetate and butyrate will last longer decaying at a slower rate, however there is considerable weight loss after three months17.
Depolymerase enzymes may be excreted by an organism so that the resulting monomers or dimers may be assimilated into the cell as a nutrient. Thermal degradation results mainly in the dimer ( 20 -38%), some trimer and small amounts of crotonic and iso-crotonic acids. A hydrolytic attack of the ester linkages results in the monomeric hydroxy-acid and is fairly rapid degrading, for example, a bottle is fully degraded within a few months. There are various alternatives to extending the time frame of degradation for example PHBV blend with 75% Cellulose, acetate and butyrate will last longer decaying at a slower rate, however there is considerable weight loss after three months17.
Since ester linkages improved the degradability of polymers, synthetic polymers were studied by one of our own students as a doctoral thesis in 199716. In her studies A. E. Taylor characterized Carboxyl - terminated and Glycidyl-terminated prepolymers which were further lengthened with Trimethyl propane and Xylitol. Ethylene glycol initiation of poly( Caprolactone) produced prepolymers which were further increased in MW by condensing with Trimethyl propane and Xylitol to exhibit a variety of properties based on MW and/or incorporation of various substrates such as Lactide and Glycolide. These ter-polyesters are proposed to have a two step degradation where the after the hydrolytic cleavage from the condensers the rate is dependent on the ter-polyester composition. These studies indicate a molecular wt tenfold less than those found in biological systems. In Biological systems the MW ranges from 20- 80 k Daltons, and varies with organisms.
The economics are such that it may be a while before bio-polyester dominate the market the similar poly propylene is 20 times cheaper to produce. Nevertheless, by virtue of their bioassimilation; human blood contains considerable amounts of PHB and many organisms as well as moist soil readily degrade the bio-polyester, they are entering the market on a regular basis, the vast and fervent research efforts indicate their viability. Moreover, bio-manufacturing yields a reliable formula for a fairly pure product once the vector is introduced. Since the insoluble PHA granules do not alter the cell biochemistry as much as 90% of the bio mass may be the desired product. The nutrients ranging from sunlight, carbon dioxide and water to gluconate, glucose etc. are renewable. Since the melting point of PHB is 1700C, the difficulty in processing is encountered, it could be overcome with engineering for different physical properties.
Biochemistry
Starting from AcetylCoA enzymatic synthesis and degradation of PHB,
the phbABCsystem8
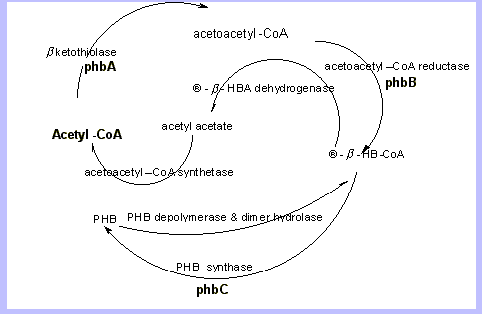
The schematic indicates the basic enzyme system employed in the synthesis as well as degradation within the cell of various micro organisms and some plants. We may differentiate among organisms and plants but the basic scheme is represented above, R. eutrophus, formerly A. eutrophus is of interest as yields of up to 90% biomass of PHB and PHBV4.
The engineering of co polymers from microorganisms and plants becomes both a lucrative field and a complex task when the variety of mechanisms and influences from regulating proteins and their respective genes are considered. For example, the phbABC scheme alone is diversified in that the operons are not necessarily present in that order or even together in the coding sequence, moreover there are modifiers such as phaF, phaG2,7,8, phaJ3, phaP18and phaR18 and of course those that are yet to be discovered which regulate the pathways.
The phaG operon encode for transacylase, an enzyme which incorporates fatty acid metabolism products into the synthesis, phaJ encodes for P(3HV) co polymerization. The codes phaP and phaR produce proteins (Phasins) which are known to regulate accumulated PHA metabolism in the cell. It is yet to be ascertained whether the difference in mechanisms in the various microorganisms or their built in tolerance or inhibition of these proteins have any effect. Some organisms may contain the genes while others function only when these are included in the vector (plasmid or cosmid). There is a growing library of these codes as research continues.
A cosmid, pVK102, introduced into A. eutrophus was able to produce 50% in body mass of PHB. With the 5.2 Kb fragment isolated, encoding the genes, when introduced into E. coli, yielded 80%10,14.
There are greater possibilities with transgenics among bacteria and yeast or even plants. 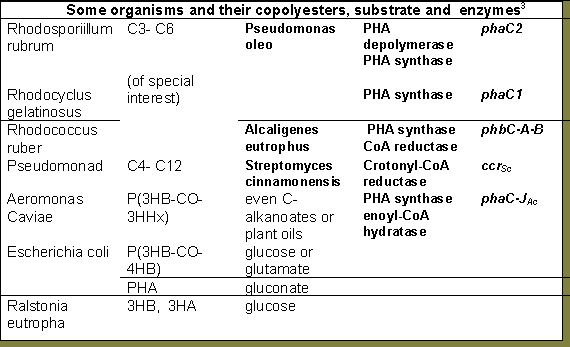 In plants the production is not as great a percentage of mass and is also limited to a growth period associated with the metabolism of the nutrients stored in the seed8. For example, while it is exciting to know that phototrophs offshore are capable of producing and accumulating PHA13, the levels change from day to night, from winter to summer and increase with appropriate nutrients, optimizing production would require considerable research into introduction of gene vectors, amplification and effects of environment.
In plants the production is not as great a percentage of mass and is also limited to a growth period associated with the metabolism of the nutrients stored in the seed8. For example, while it is exciting to know that phototrophs offshore are capable of producing and accumulating PHA13, the levels change from day to night, from winter to summer and increase with appropriate nutrients, optimizing production would require considerable research into introduction of gene vectors, amplification and effects of environment.
Changes in nutrients have a large effect on the products among the microbial world,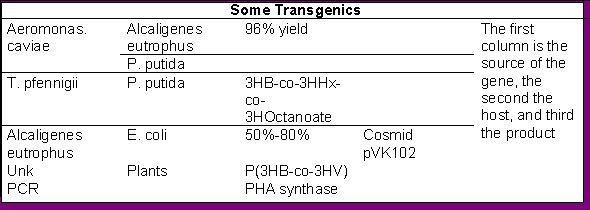 although both NADPH- and NADH-dependent acetoacetyl-CoA reductase activities have been observed in cell extracts of R. eutropha, only the former is involved in P(3HB) synthesis5. The absence of L-arabinose or the deletion of ccrSc from the plasmid resulted in accumulation of poly(3-hydroxybutyrate) homopolymer, indicating the critical role of CCR in the formation of the 3-hydroxyhexanoate unit7.
although both NADPH- and NADH-dependent acetoacetyl-CoA reductase activities have been observed in cell extracts of R. eutropha, only the former is involved in P(3HB) synthesis5. The absence of L-arabinose or the deletion of ccrSc from the plasmid resulted in accumulation of poly(3-hydroxybutyrate) homopolymer, indicating the critical role of CCR in the formation of the 3-hydroxyhexanoate unit7.
These basic schemes may be classified according to the enzyme structure necessary to assimilate the nutrients of choice, by far the Rhodobacter family which fixes carbon photo synthetically would be a favorite to research, transgenics are of interest as they adapt the synthases to their metabolism, for example with the same plasmid A. caviae is unable to use fructose as substrate, but A eutrophus is. Since the variety of metabolic schemes provide the intermediates for co polymerization the range of nutrients from which final products could be derived is expanded from sunlight, carbon dioxide and water to specific length carbon substrates such as propionate or gluconate.
While animals pose a variety of complications such as crossbreeding and developing new transmutations, or enzymatic regulation such as with phasins, plants are much more manageable and pose a far lesser risk. However the life cycle of the plants during germination when fat is catabolized seems most productive for PHB. In this regard microbial mats which have been observed to have cycles daily as well as seasonally of accumulating a considerable amount of PHB13 could prove another lucrative field for study and genetic manipulation.
Procedure3
<> So far, however, nothing compares with A. eutrophus, alias R. eutrophus, which is able to accumulate up to 90% of dry cell weight in PHAs. The life cycle is short for these microorganisms increasing the yield even more. The genes used in synthesis of P(3HB)-co-P(HHx), a copolyester with greater flexibility than the homopolymer P(3HB) and a lower melting point, are shown on the following page3. In constructing the plasmid genes from Aeromonas caviae, producer of the co polymer from alkanoates or plant oils, for the synthases phaJ and phaC are used which have been shown the authors to produce the above co polymer in E. coli from alkanoates, but with R. eutrophus the substrate is fructose.
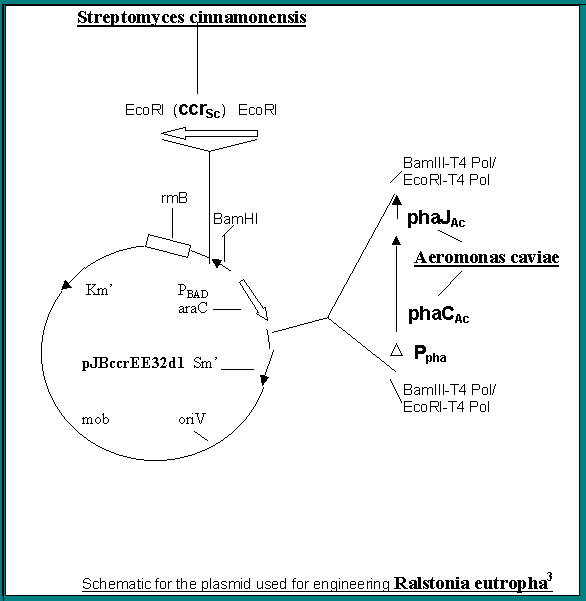
The plasmid is constructed by ligating sequences from previously constructed plasmids, the crotonyl coA reductase from Streptomyces cinnamonensis is amplified and inserted above araC. The other enzymes the two beta ketothiolases and acetoacetyl coA reductase are native to R. eutrophus. The promoter regions are important as transcription is triggered by chemicals in the cell environment. The opposite direction of the sequence may have a delayed response for its expression. Kanamycin is added at 200mg/L for maintenance of the broad-host-range plasmids. The cells are grown in 500 ml flasks shaked at 130 strokes /min for 72 hours at 300 C. ).0.5 % w/w fructose is the medium and arabinose is added after 12 hours to induce gene expression.
The composition is determined by gas chromatography after direct methanolysis of the whole dried cells, dried in the presence of 15% sulfuric acid. Extraction with chloroform for 48 h and filtration yielded the PHAs for further analysis. DSC endotherms yielded melting temperatures. 1.5 mol% P(3HHx) is incorporated and the m.p. dropped from 1750C to 1500C.
Pathway for enzymatic synthesis of P(3HB)-co-(3HHx) in R. eutrophus3
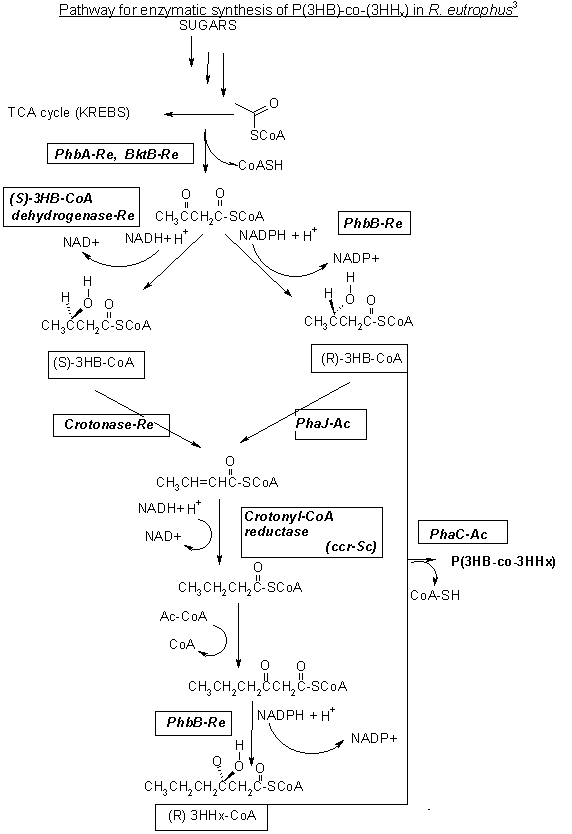
The proposed biochemical pathway for the production of the copolymer is also depicted on this page.
Discussion
The authorsRef are a foremost authority in biochemical engineering for the production of PHAs, and present a way to design polymers with requisite properties by adjusting the source of monomers. As mentioned earlier, the parameters for control are exponential when one considers transgenics, adding genes to localize production and storage in harvestable units in the plant or larger animals. Ideally, animals able to naturally secrete the product through lactation or saliva or even fecal matter are best as a steady source. The main expense comes from purification of the product.
There has been some concern via the media regarding the manipulation of genes for any purpose, and some of its negative outcomes. These are best discussed by articles cited in current issues of National Geographic1 and the Smithsonian12. Some of the effects of transgenic corn (Bt) capable of producing insecticides within the plant seems to be a source of worry when you consider the plant which kills certain caterpillars is being fed to the animals, animals which may down the line become food for us.
Nonetheless the benefits outweigh the possible pitfalls by far as micro cultivation not only is a wave of the future in terms of sheer productivity in less space and time but also as a source of a very wide variety of products.
References
-
Jennifer Ackerman,
Food How safe? How altered?,
National Geographic,
May 2002,
Vol. 201, No. 5,
p2-50.
- Silke Fiedler, Alexander Steinbüchel, and Bernd H. A. Rehm, PhaG-Mediated Synthesis of Poly(3-Hydroxyalkanoates) Consisting of Medium-Chain-Length Constituents from Nonrelated Carbon Sources in Recombinant Pseudomonas fragi, Applied and Environmental Microbiology,May 2000, Vol. 66, No. 5, p2117-24.
- Fukui T., Abe H., and Doi Y., Engineering of R. eutropha for Production of P3-HB-co-3-HHx from Fructose and Solid-State Properties of the Copolymer, Biomacromolecules. 2002, No.2, p618-24.
- Griffin G. J. L., Chemistry and Technology of Biodegradable Polymers, Blackie Academic and Professional, 1994, 48-97.
- Haywood, G. W., A. J. Anderson, L. Chu, and E. A. Dawes,The role of NADH- and NADPH-linked acetoacetyl-CoA reductases in the poly-3-hydroxybutyrate synthesizing organism Alcaligenes eutrophus, FEMS Microbiol. Lett. 1988, 52, p259-64.
- Hest, J. C. M. van, and Tirrell D. A., Protein-based materials, toward a new level of structural control, Chem. Comm., 2001, 1897-1904.
- Klinke, S.; Ren, Q.; Witholt, B.; Kessler B., Production of medium-chain-length poly(3-hydroxyalkanoates) from gluconate by recombinant Escherichia coli.
Appl. Environ. Microbiol. Feb 1999, Vol. 65, 540-8.
- Madison LL, Huisman GW., Metabolix, Inc., Cambridge, Massachusetts 02142, USA, Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic,
Microbiol Mol Biol Rev., Mar 1999 ;Vol.63(1):21-53
- Volker Mittendorf, Elizabeth J. Robertson, Rachel M. Leech, Niels Krüger, Alexander Steinbüchel, and Yves Poirier, Synthesis of medium-chain-length polyhydroxyalkanoates in Arabidopsis thaliana using intermediates of peroxisomal fatty acid -oxidation, Proc Natl Acad Sci USA, Nov 10, 1998,Vol. 95, Issue 23, 13397-402.
- Peoples, Oliver P. and Sinskey Anthony J., PHB Biosynthesis in A. eutrophus (Identification and Characterization of the PHB Polymerase Gene), J. Biol. Chem. 1989, 264, 15298.
- Yves Poirier, Giovanni Ventre, and Daniela Caldelari, Increased Flow of Fatty Acids toward -Oxidation in Developing Seeds of Arabidopsis Deficient in Diacylglycerol Acyltransferase Activity or Synthesizing Medium-Chain-Length Fatty Acids, Plant Physiology, December 1999, Vol. 121, p1359-66.
- Jim Robbins, Second Nature, Smithsonian, July 2002, Vol. 33, No. 4, p78-84.
- Rothermich, M. M., Guerrero, R., Lenz, R. W., Goodwin S., Characterization, Seasonal Occurrence, and Diel Fluctuation of Poly(hydroxyalkanoate) in Photosynthetic Microbial Mats, Appl. Environ. Microbiol. 2000, Vol. 66, p4279-91
- Slater S, Mitsky TA, Houmiel KL, Hao M, Reiser SE, Taylor NB, Tran M, Valentin HE, Rodriguez DJ, Stone DA, Padgette SR, Kishore G, Gruys KJ., Metabolic engineering of Arabidopsis and Brassica for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production., Nat Biotechnol., Oct 1999 ;17(10), p1011-6.
- Song, J. J., and S. C. Yoon. Biosynthesis of novel aromatic copolyesters from insoluble 11-phenoxyundecanoic acid by Pseudomonas putida BMO1. Appl. Environ. Microbiol. 1996, 62, p536-544.
- Taylor A. E., Synthesis and Characterization of Biodegradable Polyesters derived from Carboxylic Acid-Terminated Polyesters, Dissertation. 1997.
- Vert M., et al, Biodegradable Polymers and Plastics, Royal Society of Chemistry, 1992, 251.
- York, G. M., Stubbe, J., Sinskey, A. J.,The Ralstonia eutropha PhaR Protein Couples Synthesis of the PhaP Phasin to the Presence of Polyhydroxybutyrate in Cells and Promotes Polyhydroxybutyrate Production, J. Bacteriol. 2002, 184.
 Polyhydroxypropylate (R=H). Abbreviated P(3HP), butyrate (R=CH3), valerate (R= C2H5), hexanoate (R=C3H7) , follow as the R group becomes larger (up to 13 Carbons), abbreviated as P(3HB), P(3HV), and P(3HHx). The first poly hydroxy alkanoid (PHA) to be discovered was P(3HB) by Lemoigne in 1926 and is therefore the widely studied.
Polyhydroxypropylate (R=H). Abbreviated P(3HP), butyrate (R=CH3), valerate (R= C2H5), hexanoate (R=C3H7) , follow as the R group becomes larger (up to 13 Carbons), abbreviated as P(3HB), P(3HV), and P(3HHx). The first poly hydroxy alkanoid (PHA) to be discovered was P(3HB) by Lemoigne in 1926 and is therefore the widely studied.
 the demand for polymers increases many fold with applications in medicine ranging from surgical sutures to implants and drug delivery packaging, the list grows as more and more variety of properties may be integrated through co-polymerization.
In fact the range of products as well as substrates grows exponentially as scientists research the metabolic pathways and the variety of combinations of transgenic strains and modifications to nutrient media. For example, Song and Yoon
the demand for polymers increases many fold with applications in medicine ranging from surgical sutures to implants and drug delivery packaging, the list grows as more and more variety of properties may be integrated through co-polymerization.
In fact the range of products as well as substrates grows exponentially as scientists research the metabolic pathways and the variety of combinations of transgenic strains and modifications to nutrient media. For example, Song and Yoon Depolymerase enzymes may be excreted by an organism so that the resulting monomers or dimers may be assimilated into the cell as a nutrient. Thermal degradation results mainly in the dimer ( 20 -38%), some trimer and small amounts of crotonic and iso-crotonic acids. A hydrolytic attack of the ester linkages results in the monomeric hydroxy-acid and is fairly rapid degrading, for example, a bottle is fully degraded within a few months. There are various alternatives to extending the time frame of degradation for example PHBV blend with 75% Cellulose, acetate and butyrate will last longer decaying at a slower rate, however there is considerable weight loss after three months
Depolymerase enzymes may be excreted by an organism so that the resulting monomers or dimers may be assimilated into the cell as a nutrient. Thermal degradation results mainly in the dimer ( 20 -38%), some trimer and small amounts of crotonic and iso-crotonic acids. A hydrolytic attack of the ester linkages results in the monomeric hydroxy-acid and is fairly rapid degrading, for example, a bottle is fully degraded within a few months. There are various alternatives to extending the time frame of degradation for example PHBV blend with 75% Cellulose, acetate and butyrate will last longer decaying at a slower rate, however there is considerable weight loss after three months
 In plants the production is not as great a percentage of mass and is also limited to a growth period associated with the metabolism of the nutrients stored in the seed
In plants the production is not as great a percentage of mass and is also limited to a growth period associated with the metabolism of the nutrients stored in the seed although both NADPH- and NADH-dependent acetoacetyl-CoA reductase activities have been observed in cell extracts of R. eutropha, only the former is involved in P(3HB) synthesis
although both NADPH- and NADH-dependent acetoacetyl-CoA reductase activities have been observed in cell extracts of R. eutropha, only the former is involved in P(3HB) synthesis
